107Stories About ChemistryINDEX |
68.
Unusual Sandwiches
The number of organometallic compounds known today has by far exceeded ten thousand. But some fifteen years ago there was an annoying gap in organometallic chemistry. Chemists could find no way to implant the so-called transition metals in organic molecules. The transition metals are those arranged in the secondary subgroups of the Periodic System. The number of these metals is just under fifty. When chemists did finally succeed in preparing organic compounds of these metals, they were found to be very unstable, kinds of �organometallic freaks.� In 1951, as has happened more than once in the history of science, His Majesty Chance stepped in. The English chemist Pauson gave his student Kealy an assignment, which was anything but complicated. Kealy was to synthesize a hydrocarbon with the rather long name of dicyclopentadienyl. To do this it would be necessary to couple two five-membered carbon cycles. In other words, from two compounds with the formula C5H5 he was to obtain one: C10H8 (it was assumed that two hydrogen atoms would split off).  Kealy knew that this reaction could take place only in the presence of a catalyst, and selected ferrous chloride for the purpose. One fine morning Pauson and Kealy raised their hands in surprise. Instead of the colourless liquid expected the reaction product was beautiful orange crystals, and very stable ones to boot. They could be heated almost to 500�C, which is rather unusual in organic chemistry. But professor and student were even more surprised when they analysed the mysterious crystals, and they had reason to be. The crystals contained carbon, hydrogen and... iron. The typically transition metal iron had gone and combined with typically organic substances! The formula of this organo-iron compound also proved unusual: 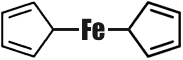 Both rings (cyclopentadienes) are flat regular pentagons, rather like two slices of bread with an iron atom between them as the filling. Compounds of this kind are now called �sandwiches�. Ferrocene (so our organo-iron compound was christened) became the first representative of the �sandwich� family. For the sake of simplicity we have depicted the structure of ferrocene schematically in a single plane; actually, its molecule possesses a more complex spacial structure. The synthesis of ferrocene was one of the greatest sensations in modern chemistry. Both theoretical and practical workers had to reconsider many of their ideas of the possibilities of organometallic chemistry, that they had hitherto thought infallible. Ferrocene was born in 1951. Today several dozen such �cenes� are known. �Sandwich� compounds have been obtained for almost all the transition metals. So far they are of interest only to theoretical chemists. As to their practical use, not everything is clear as yet. But... And now is the time to make the acquaintance of CMT. The full name of this substance is a very long one, but it is easy to remember because it rhymes: Cyclopentadienyl� And the structure of its molecule is easy to write:  Instead of the other �slice of bread� (cyclopentadienyl ring) the filling (the manganese atom) is linked with three molecules of carbon monoxide. CMT is an excellent antiknock agent, better than our old friend TEL in performance and better also because it is almost harmless. It is now undergoing practical verification from all aspects. Tank lorries with CMT printed on their sides already run the roads. Economists have figured out that complete substitution of CMT for TEL may save three billion rubles per year. But the most important advantage is that the air of our towns and cities will be cleaner and healthier. |





