107Stories About ChemistryINDEX |
65.
26, 28, or Something Quite Remarkable
These substances are called catenanes, from the Latin �catena� meaning a chain. So what of it? The word chain doesn�t tell us much. It is no less common in the organic chemist�s vocabulary than any other term. But there are chains and chains. We have had occasion to see that they may be linear or branched and sometimes form rather intricate patterns. But now stop and think a moment: in organic compounds the notion chain is a graphic, but not very strict conception. According to its everyday meaning, the word chain signifies something different. Its links have no rigid mechanical bond but pass freely through one another. In complex organic compounds the cycles are �soldered�, so to say, to one another, as, for instance, the three benzene rings in anthracene. It resembles a chain of cycles, but still it is not quite a chain. And so chemists began to puzzle over the question whether separate cycles could be connected like the links in an ordinary chain, like this:  In short, what they wanted was to connect cyclic molecules without a chemical bond, in a purely mechanical fashion, so to say. This attractive idea matured for many years in the minds of scientists Theory was on their side. It set no unsurmountable obstacles to such a synthesis. Chemists were even able to calculate theoretically the number of carbon atoms the cycles would have to consist of to be interlaceable. As regards practice, however, the situation for a long time was anything but bright. The synthesis would go into a deadlock at some stage each time And the chemists had to keep thinking up all kinds of new tricks. The new compound was born on a fine April morning in 1964. It was brought to life by two German chemists, Luttringhaus and Schill. This required twenty consecutive chemical operations, twenty stages. The compound consists of two cyclic molecules interlaced like the links of a chain. One of the links contains 26, and the other, 28 carbon atoms. Hence the prosaic name of the substance: catenane 26, 28. Two interlaced rings is now already the past of catenane chemistry. Now scientists are working on more intricate ring combinations, such as: 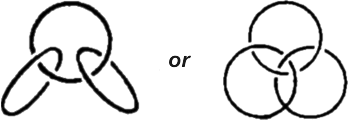 These are models of three-linked catenanes. In the one at the left the middle link must contain 26 carbon atoms, and the outer ones, 20 each. To give a complex interlacing of three rings (the catenane on the right) they must consist of at least 30 atoms each. The external appearance of the first-born in the new family, catenane 26, 28, is surprisingly commonplace: it is a white crystalline powder melting at 125�C. Do catenanes occur in nature? In nature everything is expedient; it exerts no abilities in vain. If natural catenanes do exist, there must be some specific function they fulfill. It is up to the scientists to find this out. |





