107Stories About ChemistryINDEX |
63.
A Surprise In a Simple Compound
In our days it is more than easy to learn to make photographs. Even a schoolchild can make them. He may not know all the secrets of the process (between ourselves, some of them are not known even to specialists), but to take snapshots and to develop them all he needs is a little practice and some good advice from adults. And so there is no need to go into the details of what a photographer does. He knows well, for instance, that sometimes brown spots appear on photographs, especially if they are kept in the light for a long time. The photographer could explain that they are due to underfixing of the paper (or plate). In scientific terms, this means that the plate or paper had not been held long enough in the fixing solution. What is the fixer needed for? Anyone who has ever taken the least interest in photography can answer that. It is needed to remove the silver bromide left undecomposed on film surfaces after exposure. Many different fixers have been invented. But the cheapest and most popular of them is hypo. Chemists call it sodium thiosulphate. But first a few words about sodium sulphate. It was discovered by the German chemist Johann Glauber and has been known for a very long time. That is why another name for sodium sulphate is Glauber�s salt. Its formula is Na2SO4 � 10H2O. Chemists are fond of drawing the structural formulas of compounds. They draw anhydrous sodium sulphate like this: 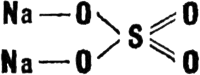 A glance at this formula makes it clear even to a greenhorn in chemistry that the sulphur in it is positively hexavalent, and the oxygen, negatively divalent. The constitution of the thiosulphate is almost the same. Except for a trifle, namely, that one of the oxygen atoms is replaced by a sulphur atom:  Simple, isn't it? But what a curious compound thiosulphate is! It contains two sulphur atoms of different valences. One of them has the change 6+, and the other 2�. It is not so very often that chemists come across such phenomena We not infrequently find the unusual in the most ordinary things. |





