107Stories About ChemistryINDEX |
60.
Chemical Rings
No few legends are told about how great scientists made their great discoveries. It is said that Newton was once absorbed in thought in his garden, when suddenly an apple fell to his feet. This gave him the clue to the law of gravity. It has been said that Mendeleyev first saw the Periodic System in his dreams. All he had to do was to wake up and put his �dream� down on paper. In a word, all kinds of stories have been thought up about discoveries and discoverers. But the idea that occurred to the famous German chemist Kekul� was really suggested by a rather curious picture. Scientists had long known about benzene, one of the most important organic compounds. They knew that it consisted of six atoms of carbon and six atoms of hydrogen. They had studied many of its reactions But they did not know the main thing, namely, how the six carbon atoms were arranged in space.  This problem gave Kekul� no peace. And here is how he solved it. Let him speak for himself: �I was at my desk writing a textbook, and was getting nowhere. My thoughts were far away. Atoms danced before my eyes. My mind�s eye could distinguish long rows of them writhing hither and thither like snakes. But lo! One of the snakes suddenly caught hold of its own tail and began rotating before my eyes as if teasing me. I started as if awakened by a stroke of lightning.� The casual image conjured up in Kekul�s mind suggested that carbon chains could close up into cycles. After Kekul� chemists began to represent the structure of benzene like this: 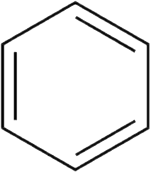 The benzene ring has played a tremendous role in organic chemistry. Rings can contain different numbers of carbon atoms. Rings may also grow together, forming weird geometrical figures. The world of rings has just as wide a range of structures as open carbon atom chains. Any book on organic chemistry bears some resemblance to a textbook of geometry, because �geometrical figures���the structural formulas of complex organic compounds�are found on almost every page. Here are two of the amusing patterns benzene rings can form  The pattern on the left is the structural formula of naphthalene. The one on the right is anthracene, a constituent of hard coal. |





